In so doing, it reduces the tendency of certain cells in the skin to release histamine.
Who is it for?
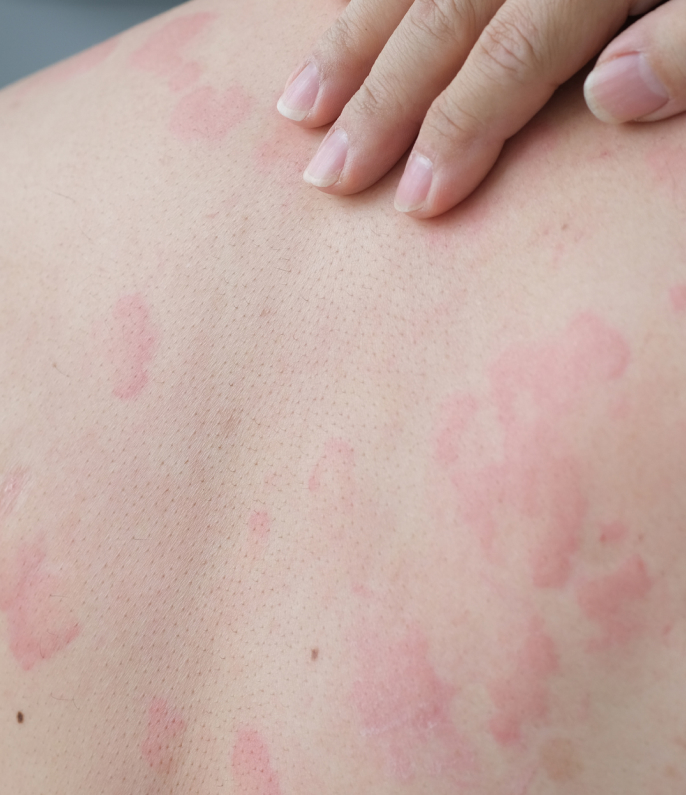
Omalizumab is used to treat patients 12-years or older who suffer with moderate-to-severe chronic spontaneous urticaria. It is also used to treat severe asthma. Patients who have failed to respond to standard treatments such as anti-H1 antihistamines (e.g: fexofenadine), anti-H2 antihistamines (e.g: famotidine) or leukotriene antagonist therapy (montelukast) may be offered omalizumab.
Please note that omalizumab is an add-on therapy, i.e. standard antihistamine should be continued whilst taking this, though antihistamine dosages can typically be reduced.
For further information about omalizumab, including a complete list of side effects, read the product and consumer medical information sheets (link below). If you have any questions about your treatment, consult your doctor.
Omalizumab is given as a subcutaneous injection (under the skin) between every 2-6 weeks. Dosing may be between 1-3 injections each time.
Omalizumab can be self-administered. Our nurses can provide assistance on how to give the injections at home.
You may notice an improvement in the itching and the rash within days after the first injection. 7-out-of-10 patients will have well-controlled urticaria by week 12 of standard dosed omalizumab. Of the remaining 3-out-of-10, around half of them will respond at week 12 to week 24. The average time to well-controlled urticaria is 6 weeks.
Omalizumab should be taken as prescribed, and you may notice recurrence of your hives if you miss doses.
Common and mild side effects include headache, abdominal pain, fever, joint pain and injection site reactions (bruising, swelling, redness, pain, warmth, stinging, itching). These are not usually severe and you should be able to continue the medication.
Less-common side effects include flu-like symptoms, heart burn, nausea, diarrhoea, worsening urticaria, cough, fainting, flushing, and increased risk of parasitic infection (patients who are planning travel or have been in areas with increased risk of parasitic infestation are advised to tell their doctor).
Anaphylaxis with angioedema is a potentially serious allergic reaction which can very rarely occur after the injection, often within the first 2 hours. You are at a higher risk of getting this type of reaction if you have had previous anaphylaxis to food or medications or if you have asthma. You should inform your doctor or nurse if you notice any of the following during or after your injection:
There is no evidence that Omalizumab increases your risk of developing cancers. Studies using Omalizumab have observed patients for up to one year after treatment.
In a study of patients with asthma, it was reported that there was a small risk of developing blood clots in the legs, lungs, heart and brain. This has not been found in studies of patients treated for chronic spontaneous urticaria.
There are no reported cases of Omalizumab interacting with local or general anaesthetic and so it is thought to be safe. However, it is important you inform your doctor or dentist that you are taking Omalizumab. The drug does not affect wound healing or increase your risk of post-operative infection.
You will typically be observed after your first injection to ensure you do not develop any signs or symptoms of an allergic reaction. No routine blood tests are required.
You will be reviewed with your treating doctor every 3-to-6 months to assess the response.
No studies have been performed in pregnant or breast-feeding women, so the effects on unborn children or babies being breast fed are unknown. Most clinical trials have stopped treatment if the participant became pregnant. Please inform your doctor if you are planning a pregnancy or become pregnant.
Omalizumab does cross the placenta and therefore the baby can be exposed to the drug.
There is no known interaction between Omalizumab and alcohol and so it is safe to drink within the nationally recommended guidelines.
Most medicines are safe to take with Omalizumab. It is important that your doctor is aware that you are having this treatment and can assess if there is a risk of drug interaction.
